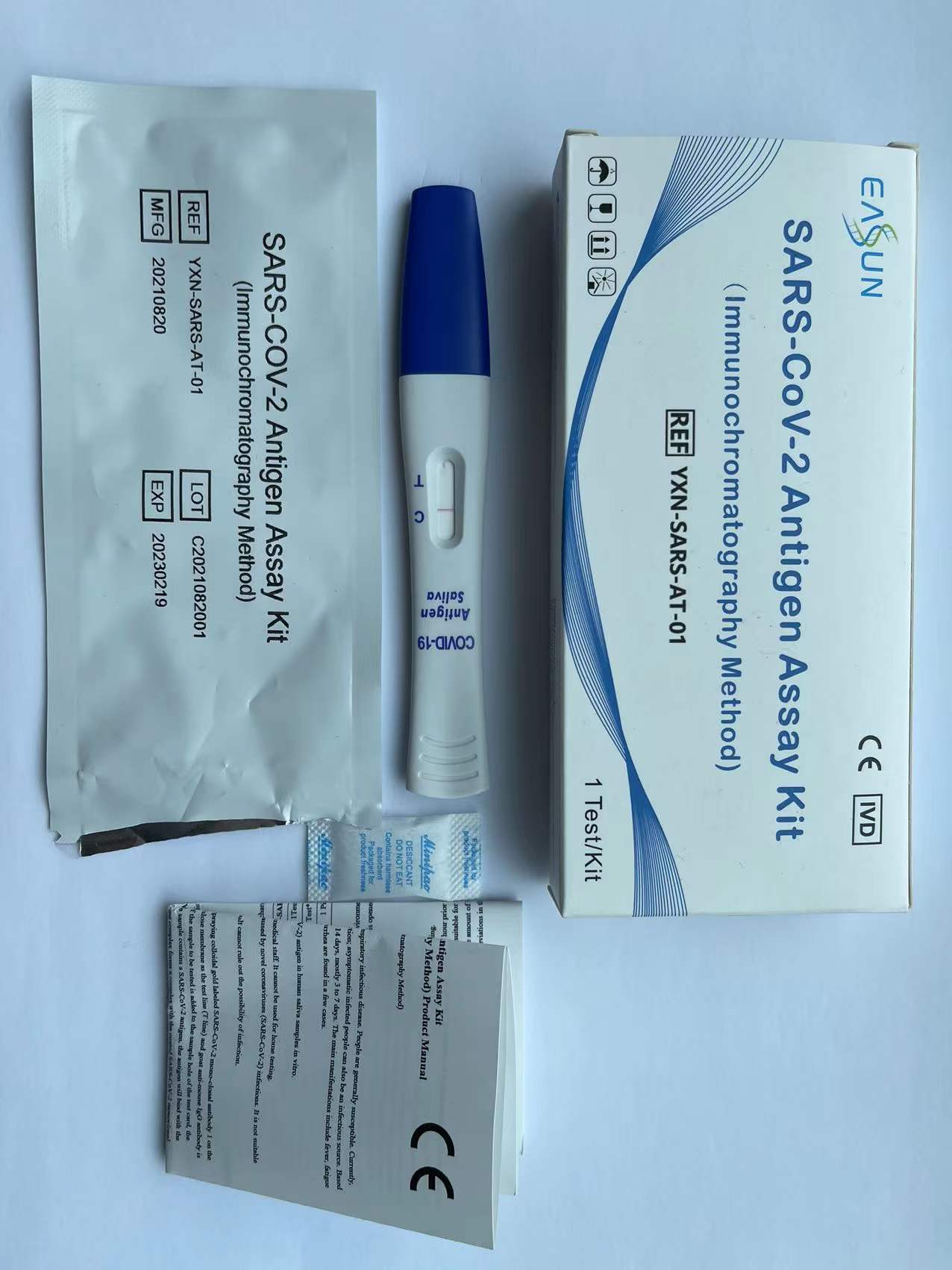
ʻO SARS-CoV-2 Antigen Assay Kit (Immunochromatography Method)
SARS-CoV-2 Antigen Assay Kit
(Immunochromatography Method) Product Manual
【PRODUCT NAME】ʻO SARS-CoV-2 Antigen Assay Kit(Ke ʻano Immunochromatography)
【PACKAGING SPECIFICATIONS】1 Hoao/Kiki
【ABSKAUKA】
Aia nā coronaviruses hou i ka genus β. ʻO COVID-19 kahi maʻi maʻi ʻeha hanu. Hiki ke maʻalahi nā kānaka. I kēia manawa, ʻo nā maʻi i loaʻa i ka coronavirus novel ke kumu nui o ka maʻi; Hiki i nā kānaka maʻi asymptomatic ke lilo i kumu maʻi. Ma muli o ka noiʻi epidemiological o kēia manawa, ʻo ka manawa incubation he 1 a 14 mau lā, ʻo ka hapa nui he 3 a 7 mau lā. ʻO nā hōʻike nui e pili ana i ke kuni, ka luhi a me ka ʻū maloʻo. Loaʻa ka nasal congestion, runny ihu, ʻeha ʻāʻī, myalgia a me ka diarrhea i kekahi mau hihia.
【EXPECTED USAGE】
Hoʻohana ʻia kēia kime e ʻike pono i ka antigen coronaviruses (SARS-CoV-2) i loko o nā laʻana kohu kanaka i loko o ka vitro. He kūpono wale ia no ka ʻoihana in vitro diagnostic, ʻaʻole no ka hoʻohana pilikino.
Hoʻohana wale ʻia kēia huahana i nā laboratories clinical a i ʻole ka hoʻāʻo koke ʻana e nā limahana lapaʻau. ʻAʻole hiki ke hoʻohana ʻia no ka hoʻāʻo home.
ʻAʻole hiki ke hoʻohana ʻia i kumu no ka maʻi a me ka wehe ʻana i ka maʻi pneumonia i hoʻokumu ʻia e nā maʻi coronaviruses (SARS-CoV-2). ʻAʻole kūpono ia no ka nānā ʻana e ka lehulehu.
Pono ka hopena ho'āʻo maikaʻi i ka hōʻoia hou ʻana, a ʻaʻole hiki i ka hopena hoʻāʻo maikaʻi ʻole ke kāpae i ka hiki ke loaʻa ka maʻi.
【PRINCIPLES OF THE PROCEDURE】
Hoʻohana kēia huahana i ka ʻenehana immunochromatography gula colloidal, kāpīpī i ke gula colloidal i kapa ʻia ʻo SARS-CoV-2 mono-clonal antibody 1 ma ka pā gula. Hoʻopili ʻia ka anti-mouse IgG antibody e like me ka laina mana maikaʻi (laina C). Ke hoʻohui ʻia ka nui kūpono o ka hāpana e hoʻāʻo ʻia i ka lua hāpana o ke kāleka hoʻāʻo, e neʻe ka hāpana ma ke kāleka hōʻike ma lalo o ka hana capillary. Inā loaʻa i ka laʻana kahi antigen SARS-CoV-2, e hoʻopaʻa ʻia ka antigen me ke gula colloidal i kapa ʻia ʻo SARS-CoV-2 monoclonal antibody 1, a ʻo ka paʻakikī paʻakikī e hana i kahi paʻakikī me ka SARS-CoV-2 monoclonal antibody 2 i uhi ʻia. ʻO ka laina T, e hōʻike ana i kahi laina T poni-ulaʻula, e hōʻike ana he maikaʻi ka antigen SARS-CoV-2. Inā ʻaʻole hōʻike ka laina hoʻāʻo T i ke kala a hōʻike i kahi hopena maikaʻi ʻole, ʻo ia ka mea ʻaʻole i loaʻa i ka laʻana ka antigen SARS-CoV-2. Aia i loko o ke kāleka ho'āʻo ka laina mana maikaʻi C, me ka nānā ʻole inā aia kahi laina hoʻāʻo, pono ke ʻike ʻia ka laina mana maikaʻi ʻulaʻula-ʻulaʻula C. Inā ʻaʻole ʻike ʻia ka laina hoʻokele maikaʻi C, hōʻike ia he hewa ʻole ka hopena hōʻike, a pono e hoʻāʻo hou ʻia kēia hāpana.
【MAIN COMPONENTS】
(1) Kāleka hoʻāʻo.
(2) Manual.
'Ōlelo Aʻo: ʻAʻole hiki ke hoʻohana ʻia nā ʻāpana o nā pūʻulu ʻokoʻa.
| Cat. No. | YXN-SARS-AT-01 |
| Package Specifications | 1 Ho'āʻo/Kiki |
| Pīkeke hoʻāʻo | 1 Hoao* 1 pack |
| Manual | 1 apana |
【STORAGE AND EXPIRATION】
ʻO ka manawa kūpono he 18 mau mahina inā mālama ʻia kēia huahana ma kahi o 2 ℃-30 ℃.
Pono e hoʻohana ʻia ka huahana i loko o 15 mau minuke ke wehe ʻia ka ʻeke pepa. Hōʻike ʻia ka lā hana a me ka lā pau ma ka lepili.
【SAMPLE REQUIREMENTS】
1. Pili i ke kanaka nasal throat swabs, oral throat swabs, saliva sample.
2. ʻOhi laʻana:
(1) ʻOhi kohu (YXN-SARS-AT-01): Hana i ka hoʻomaʻemaʻe lima me ke kopa a me ka wai / ʻona lima me ka waiʻona. Wehe i ka ipu. E hana i kahi kani Kruuua mai ka ʻāʻī e hoʻomaʻemaʻe i ka ʻāʻī mai ka ʻāʻī hohonu, a laila e kuha i ka kohu (ma kahi o 2 ml) i loko o ka ipu. E pale i ka hoʻohaumia ʻana o ke kohu o ka ʻili o waho o ka ipu. ʻO ka manawa kūpono o ka hōʻiliʻili ʻana: Ma hope o ke ala ʻana a ma mua o ka palaki ʻana i nā niho, ʻai a inu paha.
3. E hana koke i ka hāpana me ka hāpana unuhi i hāʻawi ʻia i loko o ka pahu ma hope o ka hōʻiliʻili ʻana o ka hāpana. Inā ʻaʻole hiki ke hana koke ʻia, pono e mālama ʻia ka hāpana i loko o kahi pahu plastik maloʻo, sterilized a hoʻopaʻa paʻa ʻia. Hiki ke mālama ʻia ma 2 ℃ -8 ℃ no 8 mau hola, a hiki ke mālama ʻia no ka manawa lōʻihi ma -70 ℃.
4. ʻAʻole hiki ke hoʻohana ʻia nā laʻana i hoʻohaumia nui ʻia e nā koena meaʻai waha no ka hoʻāʻo ʻana i kēia huahana. ʻAʻole ʻōlelo ʻia nā laʻana i hōʻiliʻili ʻia mai nā swabs i ʻoi aku ka viscous a i ʻole agglomerated no ka hoʻāʻo ʻana i kēia huahana. Inā haumia nā swabs me ka nui o ke koko, ʻaʻole ʻōlelo ʻia no ka hoʻāʻo ʻana. ʻAʻole ʻōlelo ʻia e hoʻohana i nā laʻana i hoʻopaʻa ʻia me ka hāʻina hoʻoheheʻe ʻana i hāʻawi ʻole ʻia i loko o kēia pahu no ka hoʻāʻo ʻana i kēia huahana.
【TESTING METHOD】
E ʻoluʻolu e heluhelu pono i ka manual ma mua o ka hoʻāʻo ʻana. E ʻoluʻolu e hoʻihoʻi i nā reagents a pau i ka wela lumi ma mua o ka hoʻāʻo. Pono e hana i ka ho'āʻo ma ka lumi wela.
Nā ʻanuʻu hoʻāʻo:
1. Laʻana kohu (YXN-SARS-AT-01):
(1) Ma hope o ka hoʻi ʻana o ka pahu hoʻāʻo i ka lumi wela, e wehe i ka ʻeke alumini alumini a lawe i waho i ka cassette hoʻāʻo a kau ʻia ma ka pākaukau.
(2) Wehe i ka piko o ka pahu ho'āʻo, e hoʻokomo i ke koʻokoʻo pahu hoʻāʻo i loko o ke kohu, a i ʻole e waiho i ka pahu hoʻāʻo ma lalo o ke alelo no 2 mau minuke.
(3) E mālama pololei i ka pahu ho'āʻo a e neʻe ka wai kohu i luna a hiki a neʻe paha ma luna o ka Laina C, a laila e hoʻihoʻi i ka poʻi a waiho i ka cassette hoʻāʻo ma ke pākaukau.
(4) E heluhelu i nā hualoaʻa i hōʻike ʻia i loko o 15-30 mau minuke, a ʻaʻole kūpono nā hualoaʻa ma hope o 30 mau minuke.
【[INTERPRETATION OF TEST RESULTS】
| ★ Hōʻike ka laina hoʻāʻo (T) a me ka laina hoʻokele (C) i nā ʻāpana kala e like me ka hōʻike ʻana o ke kiʻi ma ke ʻano pololei, e hōʻike ana he maikaʻi ka antigen SARS-CoV-2. | |
| ★NEGATIVE: Inā hoʻomohala wale ka laina hoʻokele maikaʻi C i ke kala a ʻaʻole hoʻomohala ka laina hoʻāʻo (T) i ke kala, ʻaʻole ʻike ʻia ka antigen SARSCoV-2 a maikaʻi ʻole ka hopena, e like me ka hōʻike ʻana o ke kiʻi he pololei. | |
| ★INVALID: ʻAʻole ʻike ʻia ka pūʻulu kala ma ka laina hoʻomalu maikaʻi (C), a ua hoʻoholo ʻia he hopena kūpono ʻole inā paha e hōʻike ana ka laina ʻike (T) i ka pūʻulu kala a ʻaʻole paha, e like me ka hōʻike ʻana o ke kiʻi he pololei. 'Ike 'ia. 'O ka lawa'ole ka la'ana o ka la'ana a i 'ole ka pololei 'ole o ke ka'ina hana, 'o ia ke kumu no ka hemahema o ka laina mana. |
【LIMITATION OF IKEION METHOD】
1. Hōʻoia hōʻoia
I mea e loiloi ai i ka hana diagnostic, ua hoʻohana kēia haʻawina i nā hōʻailona COVID-19-maikaʻi mai 150 mau kānaka a me nā hōʻailona COVID-19-ʻino mai 350 mau kānaka. Ua hoʻāʻo ʻia a hōʻoia ʻia kēia mau hiʻohiʻona e ke ʻano RT-PCR. ʻO nā hopena penei:
a) Hoʻonaʻauao :92.67% (139/ 150), 95%CI (87.26% , 96.28%).
b) Kūikawā:98.29% (344/350), 95%CI(96.31%, 99.37%) .
2. palena ʻike liʻiliʻi loa:
Ke ʻoi aku ka nui o ka maʻi maʻi ma mua o 400TCID50/ml, ʻoi aku ka nui o ka ʻike maikaʻi ma mua o 95%. Ke emi ka maʻi maʻi ma lalo o 200TCID50/ml, ʻoi aku ka liʻiliʻi o ka helu ʻike maikaʻi ma mua o 95%, no laila, ʻo ka palena haʻahaʻa loa o kēia huahana he 400TCID50/ml.
3. Ka pololei:
Ua hoʻāʻo ʻia ʻekolu mau pūʻulu reagents no ka pololei. Ua hoʻohana ʻia nā ʻāpana like ʻole o nā reagents no ka hoʻāʻo ʻana i ka hāpana like ʻole he 10 mau manawa i ka holomua, a ʻo nā hopena a pau ʻino. ʻOkoʻa pūʻulu o reagents ua hoʻohana 'ia e ho'āʻo i ka like maikaʻi hāpana 10 manawa i ka succession, a me ka
maikaʻi nā hopena a pau.
4. Ka hopena HOOK:
Ke hiki i ka 4.0*105TCID50/ml ka maʻiʻo maʻiʻo i loko o ka hāpana e hoʻāʻo ʻia, ʻaʻole hōʻike ka hopena hoʻāʻo i ka hopena HOOK. 5. Kea-reactivity
Ua loiloi ʻia ka hoʻololi ʻana o ka pahu. ʻAʻole i hōʻike ʻia nā hopena i ka hoʻoikaika ʻana me kēia ʻano hoʻohālike.
| No | 'ikamu | Conc | No | 'ikamu | Conc |
| 1 | HCOV-HKU1 | 105TCID50/ml | 16 | Influenza A H3N2 | 105TCID50/ml |
| 2 | ʻO Staphylococcus aureus | 106TCID50/ml | 17 | H7N9 | 105TCID50/ml |
| 3 | ʻO ka hui A streptococci | 106TCID50/ml | 18 | H5N1 | 105TCID50/ml |
| 4 | Maʻi ʻino | 105TCID50/ml | 19 | ʻO ka maʻi virus Epstein-Barr | 105TCID50/ml |
| 5 | ʻO ka maʻi maʻi mumps | 105TCID50/ml | 20 | Enterovirus CA16 | 105TCID50/ml |
| 6 | ʻO Adenovirus ʻano 3 | 105TCID50/ml | 21 | Rhinovirus | 105TCID50/ml |
| 7 | Mycoplasmal pneumonia | 106TCID50/ml | 22 | Respiratory syncytial virus | 105TCID50/ml |
| 8 | Paraimfluenzavirus, ʻano 2 | 105TCID50/ml | 23 | Streptococcus pneumoniae | 106TCID50/ml |
| 9 | ʻO ka metapneumovirus kanaka | 105TCID50/ml | 24 | ʻO Candida albicans | 106TCID50/ml |
| 10 | ʻO ka coronavirus kanaka OC43 | 105TCID50/ml | 25 | Chlamydia pneumoniae | 106TCID50/ml |
| 11 | ʻO ka coronavirus kanaka 229E | 105TCID50/ml | 26 | ʻO Bordetella pertussis | 106TCID50/ml |
| 12 | ʻO Bordetella parapertusis | 106TCID50/ml | 27 | Pneumocystis jiroveci | 106TCID50/ml |
| 13 | ʻO ka maʻi Influenza B Victoria | 105TCID50/ml | 28 | Mycobacterium tubercu losis | 106TCID50/ml |
| 14 | ʻO ka maʻi maʻi maʻi B Y | 105TCID50/ml | 29 | Legionella pneumophila | 106TCID50/ml |
| 15 | Influenza A H1N1 2009 | 105TCID50/ml |
6. Na mea keakea
ʻAʻole e hoʻopilikia ʻia nā hopena hōʻike i ka mea ma ke ʻano aʻe:
| No | 'ikamu | Conc | No | 'ikamu | Conc |
| 1 | Koko holookoa | 4% | 9 | Mucin | 0 50% |
| 2 | Ibuprofen | 1mg/ml | 10 | Huihui Benzoin Gel | 1.5mg/ml |
| 3 | ʻo ka tetracycline | 3ug/ml | 11 | Cromolyn glycate | 15% |
| 4 | chloramphenicol | 3ug/ml | 12 | Deoxyepinephrine hydro chloride | 15% |
| 5 | Erythromycin | 3ug/ml | 13 | Afrin | 15% |
| 6 | ʻO Tobramycin | 5% | 14 | Fluticasone propionate spray | 15% |
| 7 | Oseltamivir | 5mg/ml | 15 | menthol | 15% |
| 8 | Holo ʻo Naphazoline Hydrochlo i ka Nasal Drops | 15% | 16 | ʻO Mupirocin | 10mg/ml |
【LIMITATION OF IKEION METHOD】
1. Hāʻawi wale ʻia kēia huahana i nā laboratories clinical a i ʻole nā limahana lapaʻau no ka hoʻāʻo koke ʻana, ʻaʻole hiki ke hoʻohana ʻia no ka hoʻāʻo ʻana i ka home.
2. He mea kūpono wale nō kēia huahana no ka ʻike ʻana i nā mea huna o ka ihu kanaka a i ʻole ka puʻu. ʻIke ia i ka maʻi maʻi maʻi i loko o ka laʻana extract, me ka nānā ʻole i ka maʻi maʻi. No laila, ʻaʻole hiki ke hoʻopili ʻia nā hopena hoʻāʻo o kēia huahana a me nā hopena moʻomeheu virus o ka laʻana like.
3. Pono e ho'iho'i 'ia ke kāleka ho'ā'o a me ka hāpana unuhi o kēia huahana i ka mahana wela ma mua o ka ho'ohana 'ana. Hiki i ka wela kūpono ke kumu i ka hopena ho'āʻo ʻino.
4. I ka wā o ka hoʻāʻo ʻana, ʻaʻole like nā hopena hōʻike i nā hopena lapaʻau ma muli o ka lawa ʻole o ka hōʻiliʻili laʻana o nā swabs sterile a i ʻole ka hōʻiliʻili kūpono ʻole a me ka hana unuhi ʻana.
5. I ka wā o ka hoʻohana ʻana i kēia huahana, pono ʻoe e hahai pono i nā pae hana o ka manual. Hiki i nā hana hana kūpono ʻole a me nā kūlana kaiapuni ke kumu i nā hopena hōʻike ʻokoʻa.
6. Pono e hoʻololi ʻia ka swab ma kahi o 10 mau manawa ma ka paia o loko o ka paipu hoʻāʻo i loaʻa ka hāpana hoʻoheheʻe. ʻO ka liʻiliʻi a i ʻole ka nui o nā hoʻololi ʻana ke kumu i nā hopena hōʻike ʻokoʻa.
7. ʻAʻole hiki i ka hopena maikaʻi o kēia huahana ke kāpae i ka hiki ke loaʻa nā pathogens ʻē aʻe.
8. He hopena ho'āʻo maikaʻi ʻole inā ʻaʻole hiki i kēia huahana ke hōʻole i ka maikaʻi o nā pathogens ʻē aʻe.
9. Manaʻo ʻia nā hopena hōʻike maikaʻi ʻole e hōʻoia me ka nucleic acid detection reagents e pale aku i ka pilikia o ka hoʻāʻo ʻana.
10. Loaʻa paha nā ʻokoʻa o nā hopena hoʻokolohua ma waena o nā laʻana lāʻau lapaʻau hau a me nā laʻana lāʻau lapaʻau hou i hōʻiliʻili ʻia.
11. Pono e ho'āʻo koke ʻia ka mea hoʻohālike ma hope o ka hōʻiliʻili ʻana i mea e pale aku ai i nā hopena hoʻokolohua like ʻole ma hope o ka waiho lōʻihi ʻana.
12. I ka wā o ka hoʻohana ʻana i kēia huahana, pono ka nui o ka laʻana kūpono, ʻoi aku ka liʻiliʻi a i ʻole ka nui o ka nui o ka laʻana i hiki ke hōʻeha i nā hopena hoʻokolohua. Manaʻo ʻia e hoʻohana i ka pipette me ka nui o ka laʻana no ka hōʻike hoʻohui.
【PRECAUTAIONS】
1. E ʻoluʻolu e hoʻohālikelike i ka hāpana diluent a me ka kāleka hōʻoia i ka lumi wela (ma luna o 30min) ma mua o ka hoʻāʻo.
2. Pono e hoʻokō ʻia ka nānā ʻana e like me nā kuhikuhi.
3. Pono e unuhi ʻia ka hopena i loko o 15-30min, a ʻaʻole kūpono ka hopena i heluhelu ʻia ma hope o 30 min.
4. Pono e noʻonoʻo ʻia ka laʻana hoʻāʻo e like me kahi mea maʻi maʻi, a pono e hana ʻia ka hana e like me nā kikoʻī hana o ka hale hana maʻi infectious, me nā hana pale a me ka nānā ʻana i ka hana bio-safety.
5. Aia kēia huahana i nā mea holoholona. ʻOiai ʻaʻole ia e lele, pono e mālama ʻia me ka akahele i ka wā e lawelawe ai i nā kumu kumu o ka maʻi. Pono nā mea hoʻohana e hana i nā hana palekana e hōʻoia i ka palekana o lākou iho a me nā poʻe ʻē aʻe.
6. Hoʻohana ʻia nā kāleka hoʻāʻo i hoʻohana ʻia, nā mea hoʻohālike, a me nā mea ʻē aʻe e like me nā ʻōpala bio-medical ma hope o ka hoʻāʻo ʻana, a holoi i kou mau lima i ka manawa.
7. Ina ua kahe ka la'ana o keia huahana i ka ili a me ka maka, e holoi koke me ka wai nui, a e imi i ka lapaau ina pono.
8. Mai hoʻohana i ka pahu me ka poino maopopo, a me ke kāleka hōʻoia me ka pōʻai pōʻino.
9. He huahana hoʻohana hoʻokahi kēia huahana, e ʻoluʻolu, mai hoʻohana hou, a mai hoʻohana i nā huahana pau.
10. E pale pono i ka lā a me ka puhi pololei ʻana mai nā pā uila i ka wā e hoʻāʻo ai.
11. ʻAʻole hiki ke hoʻohana ʻia ka wai kīwī, ka wai i hoʻoheheʻe ʻia a i ʻole ka wai deionized a me nā mea inu e like me nā reagents mana ʻino.
12. Ma muli o ka ʻokoʻa o nā laʻana, ʻoi aku ka māmā a i ʻole ke kala hina o kekahi mau laina hoʻāʻo. Ma ke ʻano he huahana qualitative, ʻoiai aia kahi hui ma ke kūlana o ka laina T, hiki ke hoʻoholo ʻia he maikaʻi.
13. Inā maikaʻi ka hoʻāʻo, pono e hoʻohana i kēia kāleka hōʻike e nānā hou i hoʻokahi manawa e pale i nā hanana liʻiliʻi liʻiliʻi.
14. Aia kahi desiccant i loko o ka ʻeke alumini alumini, mai lawe waha